eCRF
Create, capture, and manage study data more easily than ever before – intuitive, flexible, and compliant with data protection regulations.
What is an eCRF?
The case report form 'CRF' – or in digital form the 'eCRF' (Electronic Case Report Form) – is a central study document for recording all examination data in a clinical trial or PMCF activity.
The CRF forms the backbone of result documentation. Errors, data loss, or inconsistencies can have serious consequences. An electronic CRF helps to avoid or detect such risks at an early stage.
Therefore, it is crucial that an eCRF:

is well-structured and intuitive to use

ensures secure data storage

guarantees high data quality
Rethinking eCRF: Simple, flexible, secure.
MaganaMed simplifies creation, collection, and management – compliant and intuitive.
Create eCRFs with ease
Quickly and efficiently design the perfect input form.

Drag & Drop CRF Designer – visual creation of eCRFs

Variety of field types – 12 question types such as multiple choice

Multilingual eCRFs – ideal for international studies

Automatic calculations – e.g. BMI or time intervals

Reusability – copy questions and entire forms across studies
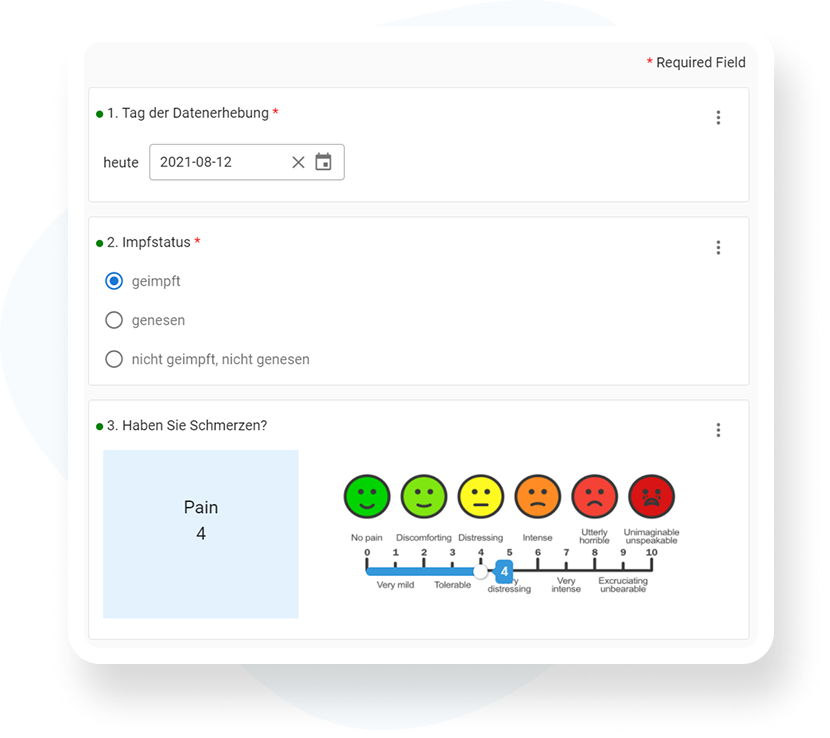
Capture data intuitively
Optimal support for study staff – clear, efficient, traceable.

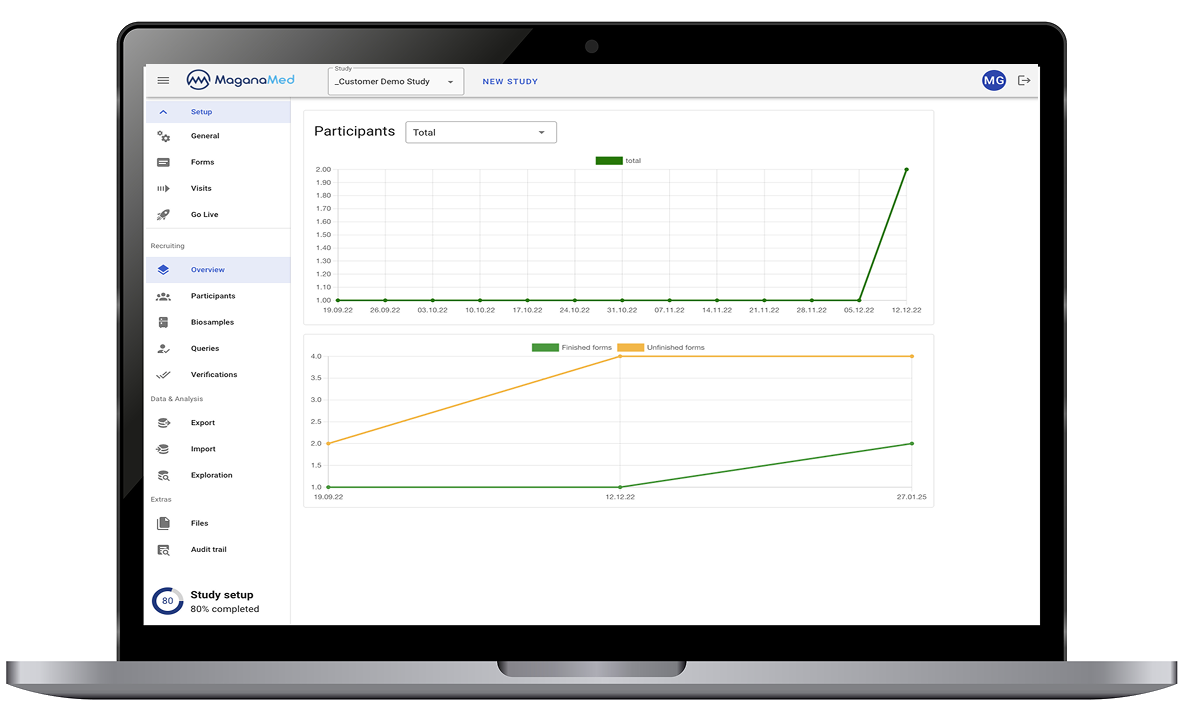
Real-time data overview – full visibility of all entries at any time

Data status: complete, incomplete, missing

Intuitive query management – fast clarifications

Data consistency – through flexible validation rules and structured input

Security & Control
Data protection and traceability at the highest level.

GDPR & GCP compliant – meets all legal requirements

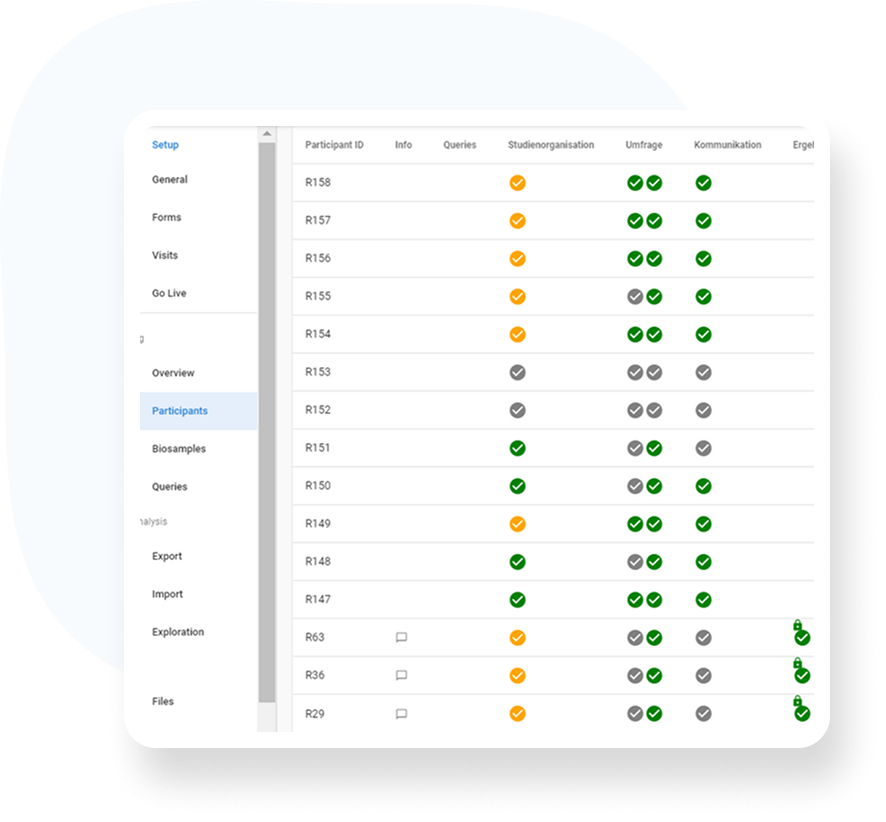
Audit trail – every change is transparently documented

Data sovereignty – export only by authorized persons

Secure storage – encrypted in ISO 27001-certified data centers (Germany)

ecrf.security.item5
Export & Documentation
Secure PDF export for seamless study documentation.

PDF export of eCRFs – for archiving or communication

Study-wide overviews – accessible at any time, even during data collection
